MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm



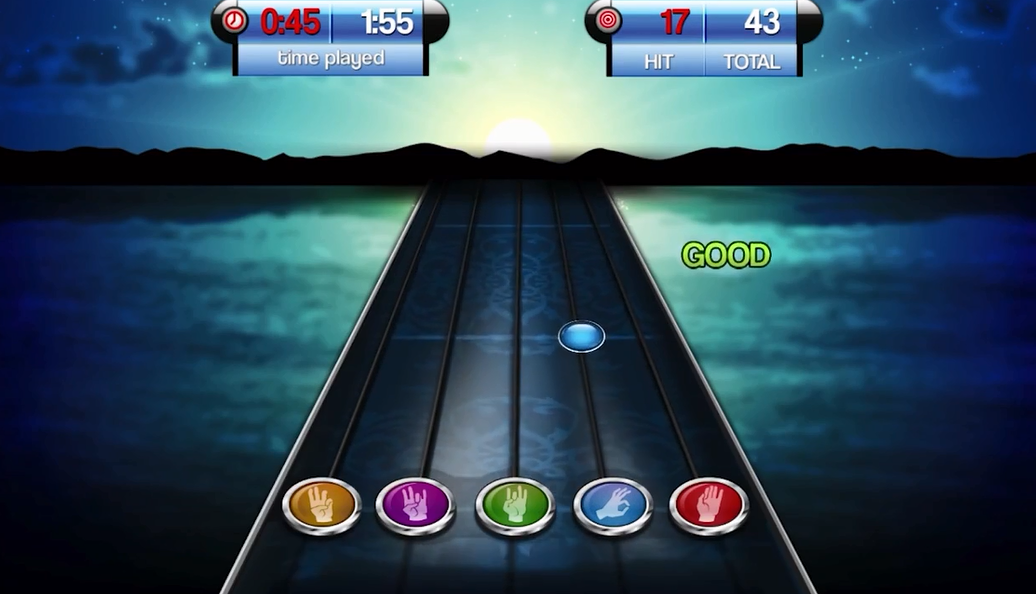


MusicGlove音乐康复治疗手套诊所版套件
MusicGlove音乐康复治疗手套概述
MusicGlove音乐康复治疗手套,包括有基于康复治疗的音乐游戏和可穿戴的感应手套,通过神经康复疗法,帮助患者改善手部活动功能的一种康复设备。MusicGlove由FDA(美国食品药品管理局)列出。
手部康复治疗的核心在于经常性重复性的手部运动,中风患者离开医院后,在没有激励的情况下,往往对反复做同样的训练动作产生厌倦。MusicGlove音乐康复治疗手套将枯燥乏味难以为继的恢复性运动治疗,通过音乐游戏的形式变得充满趣味,激发了患者主动锻炼恢复的积极性,激励患者在玩引人入胜的音乐游戏时进行数百次功能性的手指和手指的重复动作。
在每次治疗后,MusicGlove会对患者的手指精细动作进行数据记录,让治疗师能够及时掌握患者的手指运动进展情况并随时调整手部功能恢复目标和治疗方案,从而使治疗效果得以不断提升。
MusicGlove音乐康复治疗手套,使用安全,易于理解,开箱即用,无需特殊培训,适合在诊所或在家中使用。MusicGlove可满足大多数患者使用需求,包括手指残留运动很少的患者到几乎完全康复的患者,包括中风后的不懂电脑使用的老年患者也可以愉快地接受治疗。

MusicGlove音乐康复治疗手套——诊所版套件(MusicGlove Clinic Suite)
临床使用MusicGlove音乐康复治疗手套疗法的价值
- 高性价比为诊所带来更多经济效益
- 能治疗更多患者而无需增加人员
- 通过高强度训练改善临床结果
- 提供手部治疗客观的结果指标
同行评审的研究表明,MusicGlove在游戏中获得的分数与公认的手功能临床指标(例如BBT积木-箱子测验,p<0.001)显示高度关联性。
- 覆盖广大患者群体
临床研究
- 背景
- 方法
研究2:临床随机对照试验比较MusicGlove音乐疗法和传统疗法(n=12)
研究3:家庭随机对照试验比较MusicGlove音乐疗法和传统疗法(n=17)
- 结果
在第二项临床研究中,研究人员发现,对于患有中风的患者,使用MusicGlove进行6次为时一小时的治疗会比相同量的常规桌面疗法带来更大的手功能改善(蓝色柱,p = 0.01)。
在一项随机对照试验中,受试者在使用MusicGlove音乐康复治疗手套进行两周的训练后,通过BBT积木-箱子测验评分衡量,与进行传统桌面练习的参与者相比,受试者的手功能前后评估有显著的提高。
在家里使用MusicGlove进行自我指导治疗的对照研究中发现了类似的结果(橙色条形,p = 0.05)。两项研究的参与者还报告了重新获得了扣衬衫纽扣,在计算机上打字,独立进食等方面的能力。这些所取得的改进结果最有可能是由于患者在治疗期间进行了多次重复训练(> 5000)。

诊所肯定
MusicGlove音乐康复治疗手套——诊所版套件(MusicGlove Clinic Suite),得到多家知名诊所的使用及肯定,包括:
- Shirley Ryan AbilityLab(芝加哥康复研究所)
- Kennedy Krieger Institute(肯尼迪·克里格研究所)、
- Rancho Los Amigos National Rehabilitation Center(RLA国家康复中心)
有关MusicGlove音乐康复治疗手套疗法的问答
- MusicGlove适用于谁?
- 如何使用MusicGlove康复治疗手套,是否需要培训?
要使用MusicGlove音乐康复治疗手套设备,只需将MusicGlove手套穿戴在手上,将USB插头插入笔记本电脑或PC电脑或平板电脑,然后按play即可。然后,根据游戏画面和音乐,当音符球飘落在屏幕上时,跟着做适当的手指拿捏动作。
- MusicGlove有哪些歌曲可用?
- 多名患者用户可以使用同一台MusicGlove诊所版设备吗?
- MusicGlove康复治疗手套如何清洁及感控?
每副手套上的手指套部分都很容易翻转,可以使用含有酒精擦拭布或其他适合皮肤接触的消毒液擦拭布进行消毒。并要求使用的患者用肥皂洗手或用含酒精的免洗洗手液洗手。
- MusicGlove康复治疗手套尺码表有吗?
XS(特小号):11.2mm-13.9mm;S(小号):13.4mm-17.8mm;M(中号):17.8mm-21.4mm; L(大号):20.7mm-25.9mm
MusicGlove音乐康复治疗手套基于纺织品制成,主要由以下几部分材料组成
- 传感器:Nora LX导电金属化织物(镀镍/镀铜平纹织物)
- 手指套外部、手掌外部和腕带:F-56(尼龙氨纶经编织光泽针织物)
- 指套(内部):Tek Air 70(尼龙防污防水平纹织物)
- 指带和手掌内部:0.5毫米氯丁橡胶(覆盖有L泡沫尼龙针织面料)
- 缝纫线和小配饰:Gutermann Mara 100涤纶线,Gutermann Tera 40涤纶线,1/2英寸编织弹性体,Velcro品牌聚酯魔术贴斜带和缝合带
- 电子元件:使用带USB插头的A型线缆将数据信息从传感器传递到附有软件的平板或PC/MAC电脑。此过程中使用的电源为5V / 300mA。

供诊所临床使用的MusicGlove音乐康复治疗手套诊所版套件选项
- 适用于PC或Mac电脑的MusicGlove音乐康复治疗手套诊所版套件,型号:FRD-MGCS
- 带10英寸触摸屏平板电脑的MusicGlove音乐康复治疗手套诊所版便携式套件,型号:FRD-MGC10
- 带21英寸平板电脑工作站的MusicGlove音乐康复治疗手套诊所版便携式套件,型号:FRD-MGC21
- 其他附加产品——MusicGlove音乐康复治疗手套歌曲扩展包-流行歌曲,型号:FRD-MGSE-POP
- 其他附加产品——MusicGlove音乐康复治疗手套诊所版软件(2个诊所版软件额外安装许可),型号:FRD-MGA-CS
文献索引:
- 33rd Annual International Conference of the IEEE EMBS(第33届IEEE生物医学工程学会国际年会), Boston, Massachusetts USA, August 30 - September 3, 2011——《MusicGlove: Motivating and Quantifying Hand Movement Rehabilitation by using Functional Grips to Play Music》
- Journal of NeuroEngineering and Rehabilitation(神经工程与康复杂志), Published on: 30 April 2014, ——《Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional hand therapy and isometric grip training》
- Journal of Rehabilitation Research & Development(康复研究与发展杂志), JRRD Volume 53, Number 4, 2016,——《Home-based hand rehabilitation after chronic stroke: Randomized, controlled single-blind trial comparing the MusicGlove with a conventional exercise program》
原装进口
| FRD-MGCS | MusicGlove音乐康复治疗手套诊所版套件,适用于PC或Mac | 电邮询价 |
| FRD-MGC10 | MusicGlove音乐康复治疗手套诊所版套件,带10英寸触摸屏平板电脑 | 电邮询价 |
| FRD-MGC21 | MusicGlove音乐康复治疗手套诊所版套件,带21英寸平板电脑工作站 | 电邮询价 |
| FRD-MGSE-POP | MusicGlove音乐康复治疗手套诊所版套件,歌曲扩展包-流行歌曲 | 电邮询价 |
| FRD-MGA-CS | MusicGlove音乐康复治疗手套诊所版套件,2个诊所版软件额外安装许可 | 电邮询价 |
MusicGlove音乐康复治疗手套诊所套装演示视频(1)
MusicGlove音乐康复治疗手套诊所套装演示视频(2)
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
MusicGlove Clinic Suite FDA listed neurorehab that helps improve mobility for hands, arms, core, and legs. Ready to use right out of the box, no special training required Adapts to your level of recovery, even if you have little to no mobility. Used in 300+ rehabilitation hospitals, 10,000+ homes Choose Your Console: Use your own computer 10" tablet with software 21" computer with software Engage Patients with Music-Based Hand Therapy What Is MusicGlove? MusicGlove is a hand therapy device that is clinically proven to improve hand function in 2 weeks. It works by motivating users to perform hundreds of therapeutic hand and finger exercises while playing an engaging musical game. How do you use it? To use the device, you simply put the MusicGlove on your hand, plug it into your personal laptop or Flint tablet, and press play. Then, follow along and make the appropriate pinching movements when each musical note floats down the screen. Validated in Clinical Trials Validated in Clinical Trials In a randomized controlled trial, subjects demonstrated significantly greater improvements in hand function pre- to post-assessment as measured by the Box and Blocks score after two weeks of training with the MusicGlove compared to participants that performed conventional tabletop exercises (see right). Participants also reported regaining the ability to perform functional movements with their hand, such as buttoning a button, brushing their teeth, and opening doors. The MusicGlove can also assess hand function, since the score obtained in the game correlates significantly with established clinical measures of hand function (such as the Box and Box test, p < 0.001). What Is MusicGlove? MusicGlove is an FDA listed device that includes a wearable sensorized glove and a therapy-based musical game. MusicGlove motivates patients to perform hundreds of functional hand and –nger repetitions while playing the engaging musical game. The device is gradeable to the patients’ level of impairment and tracks their progress after each therapy session. The device is safe, easy to understand, and can be used in the clinic with a therapist or at home without supervision. MUSICGLOVE FOR CLINIC USE Value of MusicGlove Therapy Improve clinical outcomes through high intensity training Two randomized controlled trials performed at the University of California, Irvine showed that people using the MusicGlove had significantly greater improvements in hand function than those who performed conventional tabletop exercises. These gains were retained at a long term follow-up. See next page for more information. Treat more patients without increasing staff MusicGlove oers a motivating alternative to conventional one-on-one therapy. The software includes a self-directed 15 to 60 minute Session Mode that can be used to increase the dose of intensive therapy each patient receives without the need for increasing staff. No additional training is required! Provide objective outcome measures for hand therapy MusicGlove also comes with a proprietary Analytics Suite that tracks and logs detailed measures of patient performance. With the touch of a button, therapists can view or print a patient’s historical data in a clear, concise format. Peer-reviewed studies have shown that the MusicGlove scores are highly correlated with established clinical measures of hand function, so these scores can also be used as an outcome measure for therapy. Reach a broad patient population MusicGlove can be used right away by any qualified patient—no custom ng is necessary. The device caters to a wide spectrum of patients, from those with little residual finger movement to those who are almost fully recovered. The diffculty and intensity of the therapy can be quickly adjusted by changing the number and type of grips required during gameplay or by selecting faster or slower songs. CLINICAL STUDIES Clinical Efficacy Background The MusicGlove is based on the latest rehabilitation research which has shown that therapy programs should: Require a large number of practice repetitions Challenge each patient at an individually appropriate level Provide detailed feedback on progress over time Use music and video games to motivate patients and stimulate neural recovery Methods Study 1: Clinical feasibility study comparing MusicGlove score to level of impairment (n = 10) Study 2: Clinical RCT comparing MusicGlove Therapy to Conventional Therapy (n = 12) Study 3: Home-based RCT comparing MusicGlove Therapy to Conventional Therapy (n = 17) Results l In the initial feasibility study of the MusicGlove, researchers found that the percentage of notes hit in the MusicGlove game is strongly correlated with functional ability. This means users can set meaningful goals with the MusicGlove and receive real rewards when they achieve them. l In the second clinical study, researchers found that 6 one-hour therapy sessions with the MusicGlove lead to significantly greater improvements in hand function than a matched dose of conventional tabletop therapy for individuals with chronic stroke(blue bars, p = 0.01). l Similar results were found in the controlled study of self-guided therapy with the MusicGlove at home (orange bars, p = 0.05). Participants in both studies also reported regaining the ability to button up a shirt, type on a computer, feed themselves independently, etc. These improvements are most likely due to the large number of repetitions (>5000) patients performed during their therapy. What is MusicGlove composed of? FABRICS MusicGlove is textile based product and is made of the following materials: FABRICS Sensors: Nora-LX Conductive Metallized Fabric (Ni/Cu plated plain weave fabric) Finger cots exterior, palm exterior and wristband: F-56 (Nylon-Spandex Tricot shiny knit fabric) Finger cots (interior): Tek Air 70 (Nylon stain resistant and uid proof plain weave fabric) Finger straps and palm Interior: .5mm Neoprene (L foam covered with a Nylon jersey knit fabric) Thread and haberdashery: Gutermann Mara 100 Polyester thread, Gutermann Tera 40 Polyester thread, 1/2” braided elastic, Polyester Bias Tape Velcro Brand Soft & Flexible Sew-On Tape ELECTRONICS MusicGlove uses elecrical signals to communicate information from the sensors to the accompanying software through a USB male-A cable. The power used in this process is 5V 300mA. FRD-MGCS MusicGlove Clinic Suite for PC/Mac only Includes six [6] Gloves (Small, Medium, Large; Left & Right) One (1) USB license w/ MusicGlove Clinic Software, Premium Headphones, Connector Cable and User’s Manual FRD-MGC21 MusicGlove Clinic Stationary Suite with 21" Workstation Includes six [6] Gloves (Small, Medium, Large; Left & Right) 21 inch touchscreen monitor w/ MusicGlove clinic software preinstalled, Premium Headphones, connector cable and User’s Manual ADDITIONAL PRODUCTS FRD-MGSE-POP Song Expansion Pack for MusicGlove - Pop Includes 12 new Pop songs – 4 for each level of difficulty. Item is provided on a USB flash drive and instructions. FRD-MGA-CS Additonal MusicGlove Clinic Software License (2 Seats) Allows for two [2] additional installations onto PC/MAC. Item is provided on a flash drive w/ preinstalled software and instructions. FAQ Frequently Asked Questions about MusicGlove Therapy: Who is the MusicGlove for? MusicGlove is intended for use by individuals with mild to moderate hand impairment a er stroke, spinal cord injury, trauma c brain injury, or other neurologic injury to improve hand dexterity and strength. A qualified user should be able to touch their thumb to the index or middle finger and release this grip by a 1/4" or more. Is any special training required? The MusicGlove is ready for use right out of the box with no special training required. The en re unit takes less than 5 minutes to set-up and is designed to be user & clinician friendly. Is this a Covered Service? MusicGlove Therapy can be billed as neuromuscular reeduca on or as a therapeu c procedure/exercise (ie: CPT codes 97112 and 97110). What songs are available? Our current playlist includes unique songs from a variety of genres (jazz, folk, pop). We are always expanding our playlist, and addi onal song packs will periodically be released. Can mul ple pa ents use the same MusicGlove device? MusicGlove will accommodate all adult hand sizes. The so ware allows an unlimited number of unique user accounts to be created, so each pa ent’s performance can be recorded individually. How is the MusicGlove cleaned? The MusicGlove is manufactured with medical grade hard wearing fabric that is an -microbial and an -fungal. Finger cots on each glove are easily reversible and can be cleaned with the included alcohol wipes or with disinfec ng wipes appropriate for skin contact. Refer to next page for the cleaning procedure. Can I purchase an extended warranty? An addi onal 1 year warranty for a total of 2 years of coverage can be purchased for 15% of the final sales price. Where was the MusicGlove developed and where is it manufactured? MusicGlove was originally developed by a team of researchers and engineers at the University of California in Irvine. The device is manufactured in Southern California. Can you provide MusicGlove Product Specifications? Glove Sizing Chart Set one of the following coins on a flat surface and place your index finger tip on it or use the ruler measurements below to determine your size. XS :Min. 11.2 mm Max. 13.9 mm S :Min. 13.4 mm Max. 17.8 mm M :Min. 17.8 mm Max. 21.4 mm L :Min. 20.7 mm Max. 25.9 mm
相关产品




 显示该栏目以下所有产品
显示该栏目以下所有产品


